
Click here to read the latest updates of this trial.
Sponsored by Teva Branded Pharmaceutical Products, R&D Inc
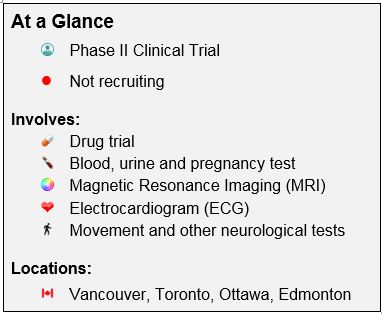 The LEGATO-HD Phase II clinical trial will examine what effect different doses of an investigational drug called laquinimod may have on people with HD. Laquinimod is an investigational drug being studied to see if it may have an effect on the symptoms of HD, including abnormal movements, memory problems, and emotional disorders. Laquinimod has already been investigated for the treatment of multiple sclerosis (MS) as it acts both on the immune system and within the central nervous system. It has been suggested that laquinimod may have an effect on the progression of HD. This trial is being conducted to determine if laquinimod helps with the signs and symptoms of HD. There are risks associated with laquinimod, and the trial physician will discuss these risks with you.
The LEGATO-HD Phase II clinical trial will examine what effect different doses of an investigational drug called laquinimod may have on people with HD. Laquinimod is an investigational drug being studied to see if it may have an effect on the symptoms of HD, including abnormal movements, memory problems, and emotional disorders. Laquinimod has already been investigated for the treatment of multiple sclerosis (MS) as it acts both on the immune system and within the central nervous system. It has been suggested that laquinimod may have an effect on the progression of HD. This trial is being conducted to determine if laquinimod helps with the signs and symptoms of HD. There are risks associated with laquinimod, and the trial physician will discuss these risks with you.
Status
This study is not currently recruiting participants. If you have any questions as a past participant, please connect with the clinical trial site directly.
Formal Name
A Multicenter, Multinational, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Laquinimod (0.5, 1.0 and 1.5 mg/day) as Treatment in Patients with Huntington’s Disease
Study Type
Interventional
Purpose
The primary objective of this study is to assess the efficacy of laquinimod 0.5, 1.0, and 1.5 mg qd in patients with HD after 12 months of treatment using the UHDRS-TMS.
Canadian Locations and Contact Information
British Columbia
Location: University of British Columbia, Vancouver
Status: Not Recruiting
Alberta
Teva Investigational Site 11124
Location: University of Alberta Hospital, Edmonton
Status: Not Recruiting
Coordinator: Paul McCann
Phone: 780-407-1614
Ontario
Teva Investigational Site 11079
Status: Not Recruiting
Location: Centre for Movement Disorders, Toronto
Coordinator: Theresa Moore
Phone: 416-847-7084
Teva Investigational Site 11118
Status: Not Recruiting
Location: Parkinson’s And neurodegenerative Disorders Clinic (1929 Russell Rd Ottawa, On K1G 4G3)
Coordinator: Neila Mendis
Phone: 613-562-4235 or 613-737-4440
Exclusion Criteria (eligibility that would exclude you from this study)
- Use of immunosuppressive agents, or cytotoxic agents, including cyclophosphamide and azatioprine within 12 months prior to screening
- Previous use of laquinimod
- Use of moderate/strong inhibitors of cytochrome P450 (CYP)3A4 within 2 weeks prior to randomization
- Use of inducers of CYP3A4 within 2 weeks prior to randomization
- Pregnant or breastfeeding
- Subjects with a clinically significant or unstable medical or surgical condition that may put the patient at risk when participating in the study or may influence the results of the study or affect the patient’s ability to take part in the study, as determined by medical history, physical examinations, ECG, or laboratory tests. Such conditions may include:
- A major cardiovascular event (e.g. myocardial infarction, acute coronary syndrome, de-compensated congestive heart failure, pulmonary embolism, coronary revascularization) that occurred during the past 6 months prior to randomization
- Any acute pulmonary disorder
- A central nervous system (CNS) disorder other than HD that may jeopardize the subject’s participation in the study, including such disorders that are demonstrated on the baseline magnetic resonance imaging (MRI) (based on local read)
- A gastrointestinal disorder that may affect the absorption of study medication
- Renal disease
- Cirrhotic patients with moderate or severe hepatic impairment
- Known human immunodeficiency virus (HIV) positive status. Patients will undergo an HIV test at screening per local requirements, if applicable
- Any malignancies, excluding basal cell carcinoma, in the 5 years prior to randomization
- Any clinically significant, abnormal, screening laboratory result which in the opinion of the investigator, affects the patients’ suitability for the study or puts the patient at risk if he/she enters the study
- Unsuitable for MRI (e.g, claustrophobia, metal implants)
- Alcohol and/or drug abuse within the 6 months prior to screening, as defined by Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition Text Revision (DSM IV TR) criteria for substance abuse
- Patients with active suicidal ideation during the past month as measured by a most severe suicide ideation score of 4 (Active Suicidal Ideation with Some Intent to Act, without Specific Plan) or 5 (Active Suicidal Ideation with Specific Plan and Intent) on the baseline screening Columbia-Suicide Severity Rating Scale (C-SSRS) or subjects who answer “Yes” on any of the 5 C-SSRS Suicidal Behavior Items (actual attempt, interrupted attempt, aborted attempt, preparatory acts, or behavior) if the attempt or acts were performed within 1 year of screening, or subjects who, in the opinion of the investigator, present a serious risk of suicide
- Patients with known intracranial neoplasms, vascular malformations, or intracranial hemorrhage
- Known drug hypersensitivity that would preclude administration of laquinimod or placebo, such as hypersensitivity to mannitol, meglumine or sodium stearyl fumarate
- Swallowing difficulties that would preclude administration of laquinimod or placebo capsules
- Treatment with any investigational product within 12 weeks of screening or patients planning to participate in another clinical study assessing any investigational product during the study.
- Patients in noninterventional and/or observational studies will not be excluded from participating in this study
- Treatment with tetrabenazine within 30 days of the study baseline visit
- Treatment with antipsychotic medication within 30 days of the study baseline visit
- Additional criteria may apply, please contact the investigator for more information
Source
ClinicalTrials.gov A service of the U.S. National Institutes of Health
This study is currently not recruiting participants.
Verified April 2015 by Teva Pharmaceutical Industries
Information provided by (Responsible Party): Teva Pharmaceutical Industries (Teva Branded Pharmaceutical Products, R&D Inc.)
ClinicalTrials.gov Identifier: NCT02215616
For more detailed information on this study and others and for the most recent updates, please go to clinicaltrials.gov ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world.
